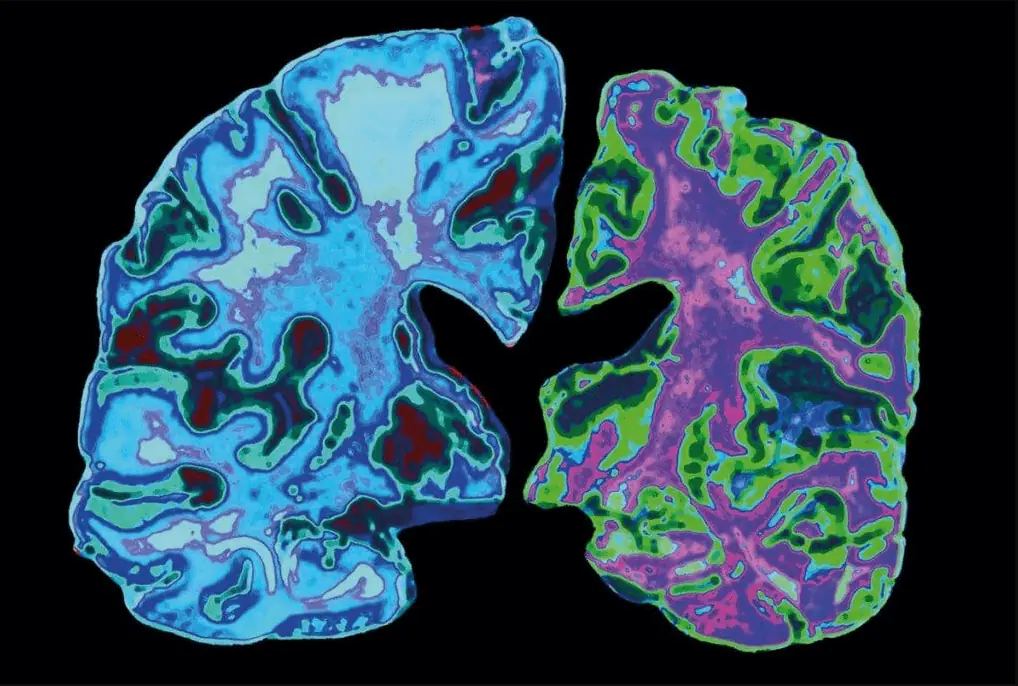
Alzheimer’s disease is a debilitating condition that affects millions of people worldwide. Despite years of research, there is still no cure for this disease. However, weighing new treatments for Alzheimer’s disease has several potential benefits that can help improve patient outcomes, reduce healthcare costs, increase research funding, improve understanding of the disease, and improve public health. In this article, we will explore each of these potential benefits in detail and discuss how weighing new treatments for Alzheimer’s disease is an important step in the fight against this debilitating condition.
Improved Patient Outcomes
Alzheimer’s disease is a progressive disorder that affects cognitive function, memory, and behavior. Currently available treatments only provide limited benefits and do not slow down the disease’s progression. However, new treatments that target the underlying mechanisms of the disease could provide more significant benefits. For instance, new drugs that reduce the production of amyloid plaques or tau protein tangles, which are the hallmark features of Alzheimer’s disease, could slow down the disease’s progression and improve cognitive function. Moreover, new treatments that target inflammation, oxidative stress, or neuronal damage could also improve patient outcomes. These new treatments have the potential to significantly improve the quality of life for individuals with Alzheimer’s disease, which is an important goal of healthcare.
Reduced Healthcare Costs
Alzheimer’s disease is a costly disease, both in terms of direct medical costs and indirect costs such as lost productivity and caregiver burden. In the United States, the annual cost of caring for individuals with Alzheimer’s disease and other dementias is estimated to be $305 billion. By 2050, this cost is projected to increase to $1.1 trillion. Developing more effective treatments that delay disease progression, reduce hospitalizations and long-term care, and improve quality of life could help reduce these costs significantly. For instance, new treatments that delay disease progression could reduce the need for hospitalization and long-term care, which are major contributors to healthcare costs. Moreover, improved quality of life for individuals with Alzheimer’s disease could reduce caregiver burden and improve productivity, which would have a positive impact on the economy.
Increased Research Funding
Despite years of research, the underlying mechanisms of Alzheimer’s disease are not fully understood. Moreover, most clinical trials of new treatments have failed to show significant benefits, leading to a decline in research funding. However, weighing new treatments for Alzheimer’s disease can help increase funding for research into the disease. New treatments that target the early stages of the disease, such as mild cognitive impairment, could lead to new discoveries and breakthroughs in treatment. Additionally, new treatments that involve personalized medicine, gene therapy, or stem cell therapy could also provide new avenues for research. Investing in research into new treatments for Alzheimer’s disease is critical for improving our understanding of the disease and developing more effective treatments.
Improved Understanding of the Disease
Weighing new treatments for Alzheimer’s disease can also lead to a better understanding of the underlying mechanisms of the disease. New imaging techniques such as positron emission tomography (PET) and magnetic resonance imaging (MRI) can help identify the changes in the brain that occur during the disease’s progression. Moreover, new genetic studies can identify the risk factors and genetic variants associated with the disease. Such insights can inform the development of future treatments and potentially even a cure. Improved understanding of the disease is critical for developing more effective treatments and ultimately finding a cure for Alzheimer’s disease.
Alzheimer’s disease is a major public health issue, affecting millions of people around the world. Moreover, the disease has a significant impact on the individuals, families, and society as a whole. Caring for individuals with Alzheimer’s disease can be emotionally and financially draining for caregivers. Moreover, individuals with Alzheimer’s disease are at increased risk of falls, injuries, and other health problems. Developing new treatments for the disease can have a significant impact on public health, reducing the burden of the disease on individuals, families, and society as a whole. Moreover, new treatments could improve quality of life and reduce healthcare costs, which would have a positive impact on the economy.
Challenges in Developing New Treatments
Developing new treatments for Alzheimer’s disease is a complex and challenging process. The disease is multifactorial, with several underlying mechanisms involved. Moreover, the blood-brain barrier presents a significant challenge in delivering drugs to the brain. Additionally, Alzheimer’s disease is a chronic condition that requires long-term treatment, which can be costly and challenging for patients and caregivers. Furthermore, clinical trials of new treatments for Alzheimer’s disease have a high failure rate, which can be discouraging for researchers and investors.
Blood-Brain Barrier
The blood-brain barrier is a protective mechanism that prevents harmful substances from entering the brain. However, this mechanism also presents a challenge in delivering drugs to the brain. Many drugs that are effective in treating other diseases are unable to cross the blood-brain barrier, limiting their efficacy in treating Alzheimer’s disease. Researchers are exploring several strategies to overcome this challenge, including nanotechnology, gene therapy, and immune system modulation.
Clinical Trial Failures
Clinical trials of new treatments for Alzheimer’s disease have a high failure rate. Many drugs that show promise in preclinical studies fail to show significant benefits in clinical trials. This high failure rate is partly due to the complexity of the disease and the lack of understanding of its underlying mechanisms. However, it is also due to the limitations of current clinical trial designs, which may not be optimal for Alzheimer’s disease. To address this issue, researchers are exploring new trial designs, such as adaptive trials, that allow for flexibility and early efficacy assessments.
Promising New Treatments
Despite the challenges in developing new treatments for Alzheimer’s disease, several promising treatments are currently under investigation.
Biogen’s Aducanumab
Aducanumab is a monoclonal antibody that targets amyloid plaques, one of the hallmark features of Alzheimer’s disease. In clinical trials, aducanumab showed a significant reduction in amyloid plaques and a slowing of cognitive decline in patients with early-stage Alzheimer’s disease. In June 2021, the FDA approved aducanumab for the treatment of Alzheimer’s disease, making it the first drug to be approved for the disease in nearly 20 years.
Leuco-methylthioninium-bis(Hydromethanesulfonate) (LMTM)
LMTM is a drug that targets tau protein tangles, another hallmark feature of Alzheimer’s disease. In clinical trials, LMTM showed a significant reduction in tau protein tangles and a slowing of cognitive decline in patients with mild-to-moderate Alzheimer’s disease. However, subsequent trials did not show significant benefits, and further research is needed to determine the drug’s efficacy.
BAN2401
BAN2401 is a monoclonal antibody that targets amyloid plaques. In clinical trials, BAN2401 showed a significant reduction in amyloid plaques and a slowing of cognitive decline in patients with early-stage Alzheimer’s disease. Moreover, BAN2401 has shown potential in reducing the accumulation of amyloid plaques in the brain, which could have significant benefits in the long term. BAN2401 is currently in phase III clinical trials, and the results are eagerly awaited.
Other Promising Treatments
Several other treatments are also under investigation, including:
- GV-971, a drug derived from seaweed that targets gut bacteria
- Amylyx Pharmaceuticals’ AMX0035, a combination therapy that targets neuroinflammation and neuronal damage
- Gene therapies that target genetic risk factors for Alzheimer’s disease, such as apolipoprotein E (APOE)
While these treatments are still in the early stages of investigation, they hold promise for the future of Alzheimer’s disease treatment.
Conclusion
Alzheimer’s disease is a devastating condition that affects millions of people worldwide. Despite the significant amount of research that has been done in the field, there is still no cure for this disease. However, weighing new treatments for Alzheimer’s disease has several potential benefits, including improved patient outcomes, reduced healthcare costs, increased research funding, improved understanding of the disease, and improved public health. New treatments that target the underlying mechanisms of the disease could provide more significant benefits than currently available treatments. While there are challenges in developing new treatments for Alzheimer’s disease, promising new treatments are currently under investigation, and the future is looking brighter for individuals affected by this devastating condition.
It is important to continue investing in research into new treatments for Alzheimer’s disease. The disease has a significant impact on public health, and developing new treatments could reduce the burden of the disease on individuals, families, and society as a whole. Moreover, new treatments could improve quality of life and reduce healthcare costs, which would have a positive impact on the economy. We must continue to support researchers and healthcare providers in their efforts to develop more effective treatments for Alzheimer’s disease.